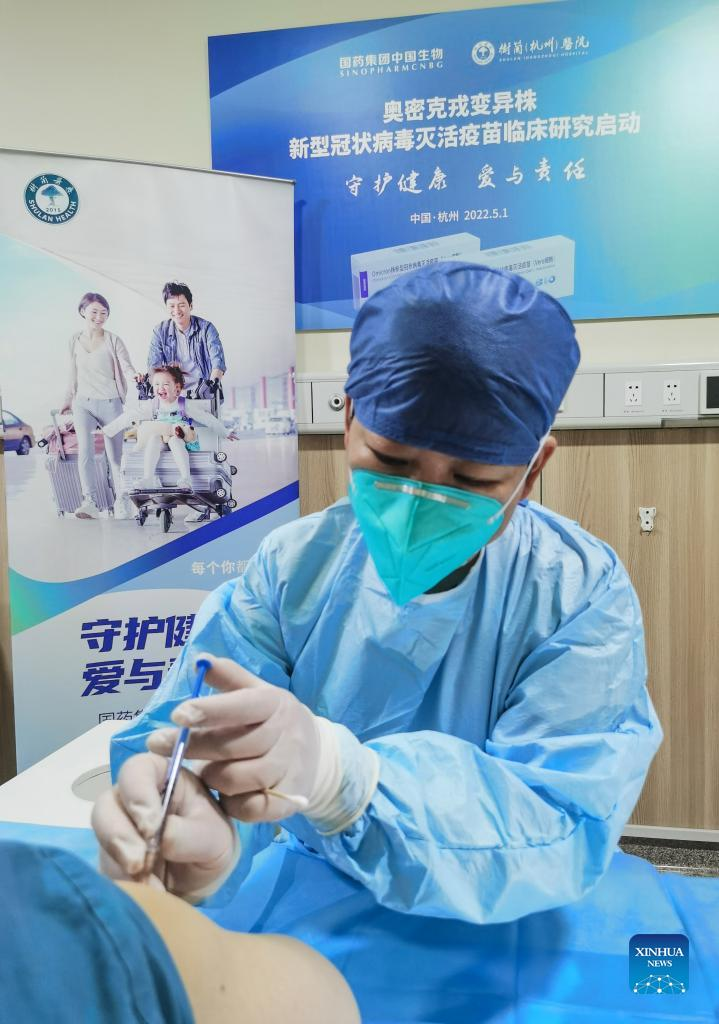
A clinical trial volunteer receives a dose of China's inactivated vaccine against Omicron variants in Hangzhou, east China's Zhejiang Province, May 2, 2022. The inactivated vaccine against Omicron variants developed by the China National Biotec Group affiliated with Sinopharm was approved for clinical trials by the National Medical Products Administration on April 26. The clinical trials are expected to conclude in three to four months and will be gradually put into use. (Xinhua)
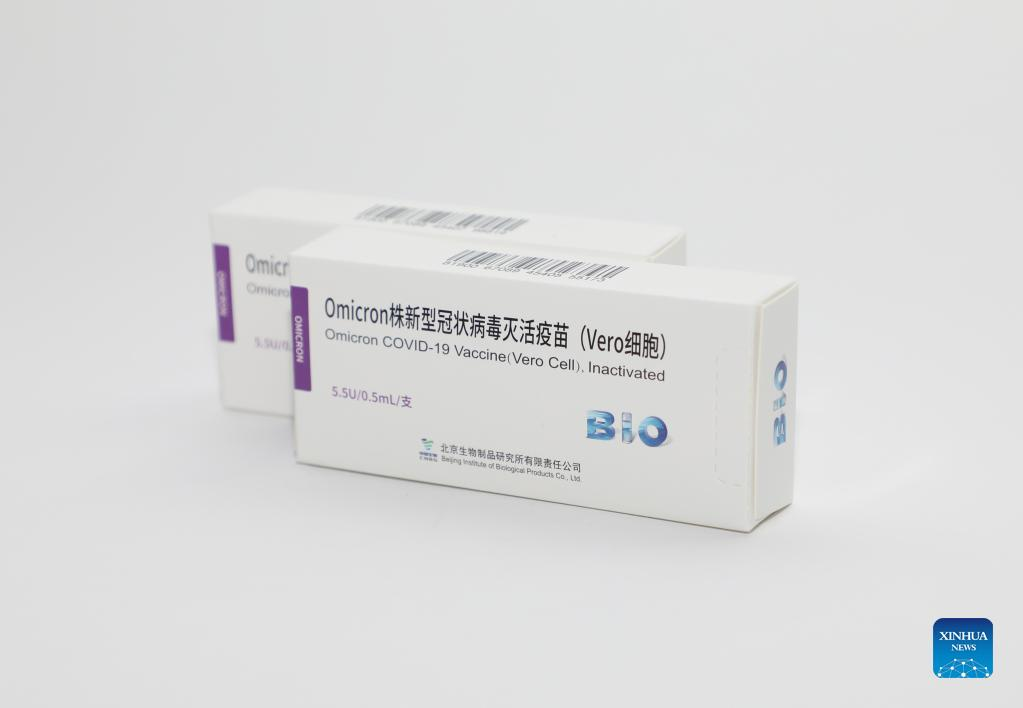
Photo taken on April 22, 2022 shows the inactivated vaccine against Omicron variants developed by the China National Biotec Group affiliated with Sinopharm. The inactivated vaccine against Omicron variants developed by the China National Biotec Group affiliated with Sinopharm was approved for clinical trials by the National Medical Products Administration on April 26. The clinical trials are expected to conclude in three to four months and will be gradually put into use. (Xinhua)
A clinical trial volunteer receives a dose of China's inactivated vaccine against Omicron variants in Hangzhou, east China's Zhejiang Province, May 1, 2022. The inactivated vaccine against Omicron variants developed by the China National Biotec Group affiliated with Sinopharm was approved for clinical trials by the National Medical Products Administration on April 26. The clinical trials are expected to conclude in three to four months and will be gradually put into use. (Xinhua)
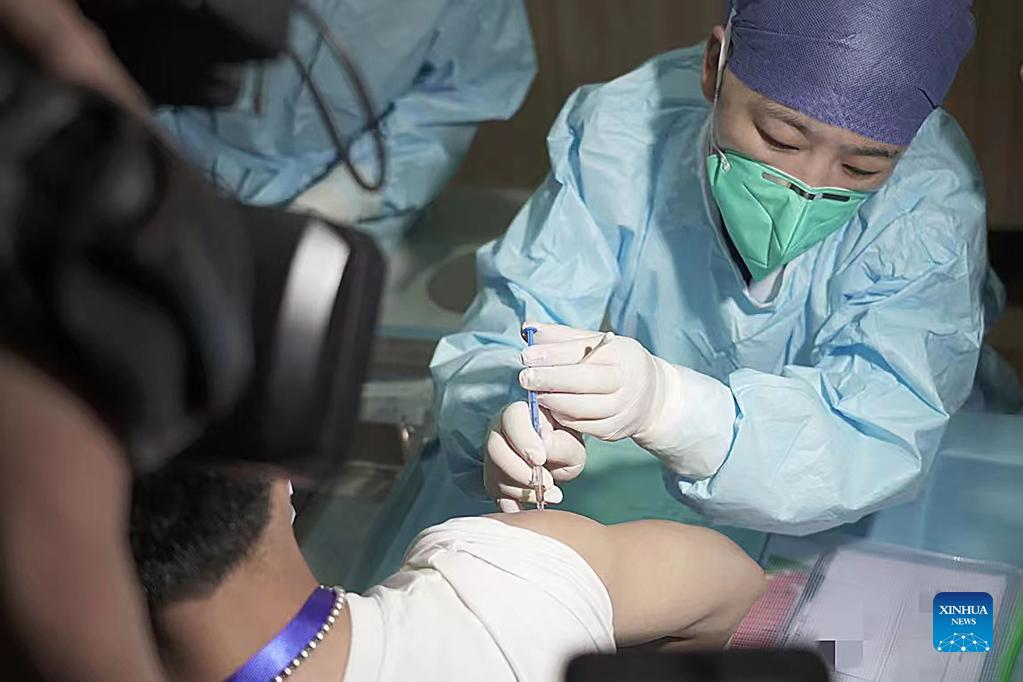
A clinical trial volunteer receives a dose of China's inactivated vaccine against Omicron variants in Hangzhou, east China's Zhejiang Province, May 1, 2022. The inactivated vaccine against Omicron variants developed by the China National Biotec Group affiliated with Sinopharm was approved for clinical trials by the National Medical Products Administration on April 26. The clinical trials are expected to conclude in three to four months and will be gradually put into use. (Xinhua)



