A technician operates during a test run of the "fill and finish" process for CanSino vaccine at Solution Biologics factory in Kuala Lumpur, Malaysia, Sept. 8, 2021. (Photo by Chong Voon Chung/Xinhua)
It will be possible to fill and finish some 3 to 6 million doses of vaccines per month when production starts, with Malaysian pharmaceutical company Solution Biologics aiming to work hand in hand with China's CanSino to tap into the ASEAN market and the world market as well.
KUALA LUMPUR, March 7 (Xinhua) -- The close cooperation between China and Malaysia in vaccine production bodes well for COVID-19 vaccine supply for Malaysia and the region, said drugmakers working on finalizing the localized fill and finish for Chinese company CanSino Biologics' COVID-19 vaccine.
The single-dose vaccine, based on the adenovirus platform, will complement other Chinese vaccines being used in the Association of Southeast Asian Nations (ASEAN), especially as booster shots, Malaysian pharmaceutical company Solution Biologics deputy group managing director Mohd Nazlee Kamal told Xinhua in a recent interview.
Mohd Nazlee said it will be possible to fill and finish some 3 to 6 million doses of vaccines per month when production starts, with Solution Biologics aiming to work hand in hand with CanSino to tap into the ASEAN market and the world market as well.
Malaysia approved the use of the CanSino COVID-19 vaccine in June last year, adding it to its vaccine portfolio alongside Sinovac COVID-19 vaccine and Sinopharm COVID-19 vaccine.

Photo shows the test run of the "fill and finish" process for CanSino vaccine at Solution Biologics factory in Kuala Lumpur, Malaysia, Sept. 8, 2021. (Photo by Chong Voon Chung/Xinhua)
In February, Solution Biologics received good manufacturing practices approval from the National Pharmaceutical Regulatory Agency to formulate and locally fill and finish the CanSino COVID-19 vaccine.
Several countries in ASEAN also use Chinese developed vaccines as part of their national immunization programs, including Indonesia, Thailand, Cambodia and the Philippines.
CanSino Biologics external R&D Vice President Xin Chunlin said both the injectable CanSino vaccine and its inhaled form, which is undergoing trials, would allow a more efficient vaccination for the public.
He said the inhaled form of the vaccine is more flexible, using one-fifth of the formulation of the injectable version, and can stay stable for almost a month under room temperature.
Xin, together with Wang Hongyi, vice president of CanSino's international business, has visited several countries including Mexico, Pakistan, Malaysia and Indonesia, countries which Wang said were eager to cooperate with China in obtaining the technology.
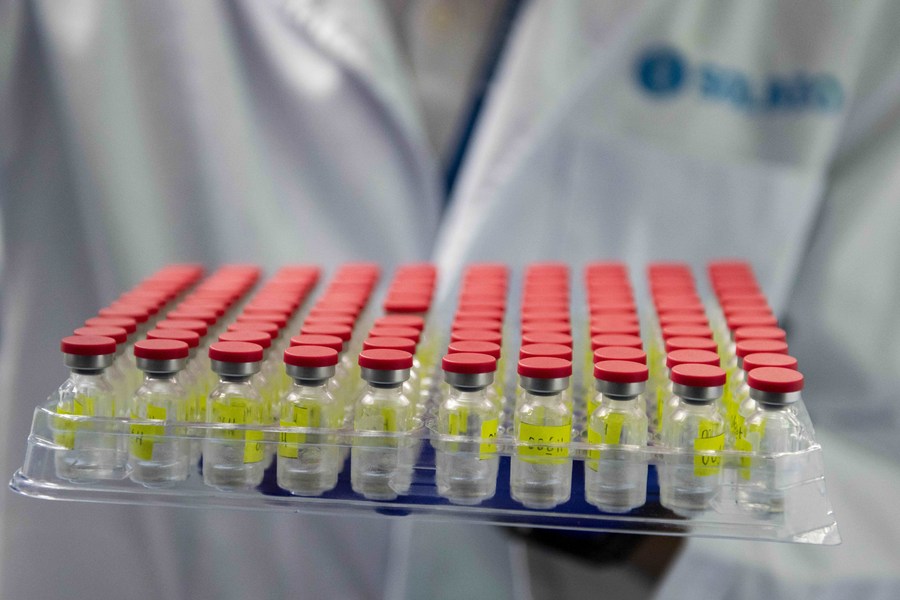
A technician operates during a test run of the "fill and finish" process for CanSino vaccine at Solution Biologics factory in Kuala Lumpur, Malaysia, Sept. 8, 2021. (Photo by Chong Voon Chung/Xinhua)
Elaborating on the significance of localized fill-and-finish production of adenovirus-based vaccines in Malaysia, Genome and Vaccine Institute director Ghows Azzam said the move would be a game-changer for Malaysia, which is seeking to develop and manufacture its own vaccines.
Ghows said the close cooperation between China and Malaysia has facilitated the country in building up its vaccine capacity.
"The support from the government and of course, the good relationship with the Chinese government will definitely help us to catalyze our innovation in vaccine development," he said. ■