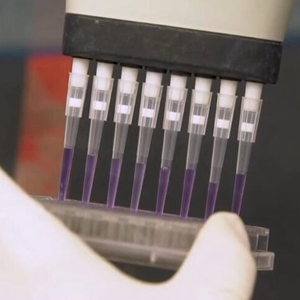
The preliminary phase III clinical studies of Chinese Sinovac's CoronaVac vaccine in Chile have shown overall safety and immunogenicity against COVID-19, according to the project team.
The clinical trials were led by Pontifical Catholic University of Chile. The project team gave their study reports to the Chilean government on Wednesday.
The scientific director of the project, Susan Bueno, discussed the team's findings at a news conference on the same day.
According to Bueno, the main conclusion drawn is that the vaccine is able to produce antibodies to help fight the virus, especially after the second dose. The interval between the doses is two to four weeks.
Meanwhile, Katia Abarca, medical director of the studies, explained that almost no side effects have been reported.
The Sinovac vaccine was approved for emergency use on Jan. 20 by the Public Health Institute of Chile.
Chilean President Sebastian Pinera on Feb. 12 received his first dose of the CoronaVac vaccine, along with those aged 71 and over.
Produced by Xinhua Global Service