A commencement ceremony for the phase 3 clinical trial of inactivated COVID-19 vaccine in the United Arab Emirates is held in Beijing, capital of China, June 23, 2020. China announced on Thursday that it had granted conditional marketing authorization for its first self-developed COVID-19 vaccine. The inactivated vaccine, which got the approval from the National Medical Products Administration (NMPA), is developed by the Beijing Biological Products Institute Co., Ltd. under the China National Biotec Group (CNBG), which is affiliated with Sinopharm. "The COVID-19 vaccines will be provided free of charge to all Chinese people," said Zeng Yixin, deputy head of the National Health Commission. (Xinhua)
BEIJING, Dec. 31 (Xinhua) -- China announced on Thursday it had granted conditional marketing authorization for the first COVID-19 vaccine.
The inactivated vaccine, which got approval from the National Medical Products Administration (NMPA), is developed by the Beijing Biological Products Institute Co., Ltd. under the China National Biotec Group (CNBG), affiliated with Sinopharm.
The interim results of its phase-3 clinical trials show 79.34 percent efficacy against COVID-19, meeting the standards of the World Health Organization and the NMPA, according to a press conference by the State Council joint prevention and control mechanism against COVID-19.
Chen Shifei, deputy head of the NMPA, said that the conditional approval was granted according to relevant laws.
The NMPA will urge the company to continue carrying out the phase-3 clinical trials as planned and ensure the research quality after the conditional approval. The company needs to report abnormal physical vaccination reactions and update the vaccine instructions in time, Chen said.
The NMPA will also strengthen the whole-chain supervision of the vaccine. It has established a traceability information system for the management of approved vaccines, Chen added.
Wu Yonglin, president of the CNBG, said the company has carried out large-scale phase-3 clinical trials in the United Arab Emirates, Bahrain, and other countries, involving 60,000 volunteers of 125 nationalities.
The United Arab Emirates and Bahrain approved the vaccine's registration according to the World Health Organization technical standards in December. More detailed data on the vaccine will be released later. It will also be published in scientific journals, Wu said.
"Data obtained in the vaccine's phase-1 and phase-2 clinical trials has shown that the antibody can maintain at a high level for more than six months. We will continue to work on the long-term protection data of the vaccine," Wu said.
China has adopted five technological approaches to developing COVID-19 vaccines. Since July, several Chinese vaccine candidates have carried out phase-3 clinical trials overseas, said Zeng Yixin, deputy head of the National Health Commission (NHC). He added that China has been at the global forefront of COVID-19 vaccine development.
"The COVID-19 vaccines will be provided free of charge to all Chinese people," Zeng said.
China approved the emergency use of COVID-19 vaccines in June, targeting groups with high risks of infection. By the end of November, more than 1.5 million doses of Chinese COVID-19 vaccines had been distributed for emergency use. Sixty-thousand vaccinated people have traveled abroad to high-risk regions, with no severe adverse reactions reported, Zeng said.
On Dec. 15, China officially launched a vaccination program for this winter-spring period targeting several key groups. These groups include those engaged in handling imported cold-chain products, customs officers, medical workers, and people working in public transport and fresh markets. In the past 15 days, the number of vaccination doses among the key groups exceeded 3 million.
"The 3 million vaccine doses, plus the previous 1.5 million doses, fully prove that Chinese vaccines are safe," Zeng noted.
After the COVID-19 vaccines are approved to enter the market, especially when production capacity increases, China will comprehensively vaccinate senior citizens, people with underlying conditions, and the general public systematically, the NHC official said.
"China will vaccinate the eligible population as widely as possible, and gradually build an immune barrier in the whole population to control the epidemic," said Zeng. He added that 60 or even 70 percent of the vaccination rate is needed to establish universal protection.
Mao Junfeng, an official from the Ministry of Industry and Information Technology (MIIT), said China is accelerating vaccine industrialization. The MIIT has coordinated with relevant enterprises to ensure a sound supply chain for vaccine production.
At present, 18 enterprises have started to increase the production capacity of COVID-19 vaccines. The Beijing Biological Products Institute Co., Ltd. under the CNBG has begun large-scale production of the approved vaccine, Mao said. He added that China's production capacity could meet the demand for mass vaccination in the country.
Xu Nanping, Vice Minister of Science and Technology, said China would continue to promote the phase-3 clinical trials of vaccines and provide more vaccines using various technological approaches.
"We will pay close attention to the virus mutation around the world and ensure that the use of vaccines is not affected. The mutations we have observed up to now will not have a substantial impact on the efficacy of the vaccines," Xu said.
China has made a specific plan and set up several research groups to tackle the possible impact of virus mutations on vaccine efficacy, Xu added. Enditem

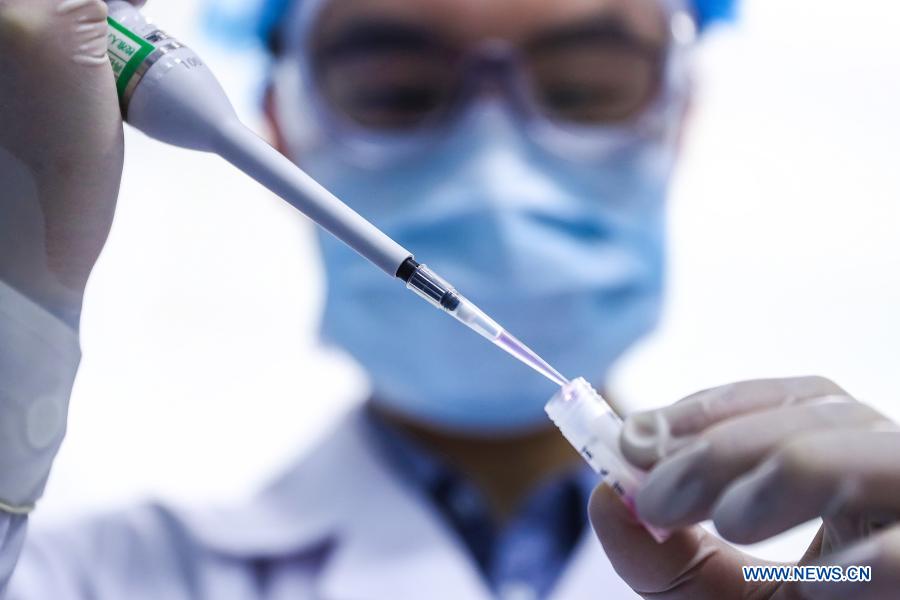
A staff member tests samples of the COVID-19 inactivated vaccine at a vaccine production base of the Beijing Biological Products Institute Co., Ltd. in Beijing, capital of China, April 11, 2020. China announced on Thursday that it had granted conditional marketing authorization for its first self-developed COVID-19 vaccine. The inactivated vaccine, which got the approval from the National Medical Products Administration (NMPA), is developed by the Beijing Biological Products Institute Co., Ltd. under the China National Biotec Group (CNBG), which is affiliated with Sinopharm. "The COVID-19 vaccines will be provided free of charge to all Chinese people," said Zeng Yixin, deputy head of the National Health Commission. (Xinhua/Zhang Yuwei)

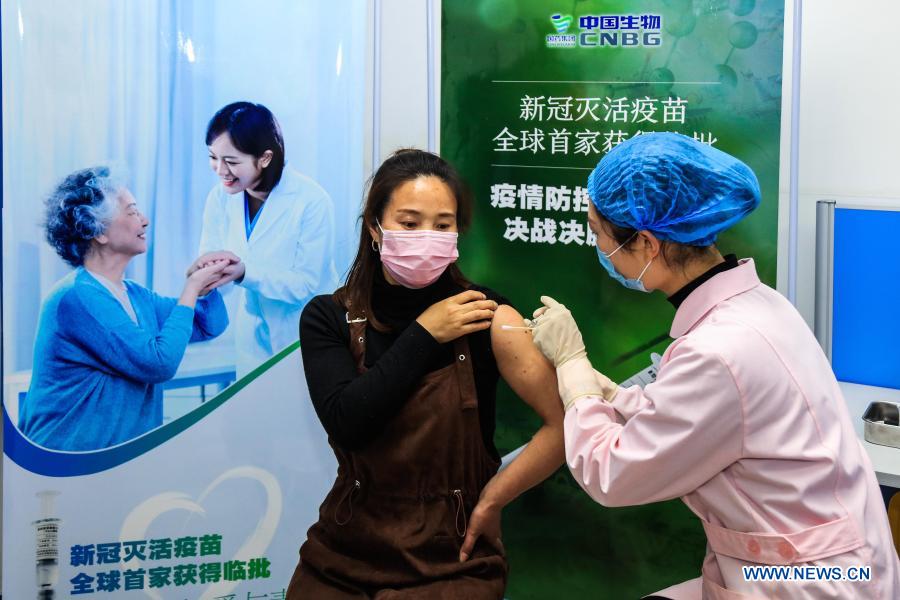
A volunteer receives the COVID-19 vaccine developed by Wuhan Biological Products Institute Co., Ltd. in Wuzhi County, central China's Henan Province, April 12, 2020. China announced on Thursday that it had granted conditional marketing authorization for its first self-developed COVID-19 vaccine. The inactivated vaccine, which got the approval from the National Medical Products Administration (NMPA), is developed by the Beijing Biological Products Institute Co., Ltd. under the China National Biotec Group (CNBG), which is affiliated with Sinopharm. "The COVID-19 vaccines will be provided free of charge to all Chinese people," said Zeng Yixin, deputy head of the National Health Commission. (Xinhua)


Staff members pose for a photo at a vaccine production base of the Beijing Biological Products Institute Co., Ltd. in Beijing, capital of China, April 10, 2020. China announced on Thursday that it had granted conditional marketing authorization for its first self-developed COVID-19 vaccine. The inactivated vaccine, which got the approval from the National Medical Products Administration (NMPA), is developed by the Beijing Biological Products Institute Co., Ltd. under the China National Biotec Group (CNBG), which is affiliated with Sinopharm. "The COVID-19 vaccines will be provided free of charge to all Chinese people," said Zeng Yixin, deputy head of the National Health Commission. (Xinhua/Zhang Yuwei)

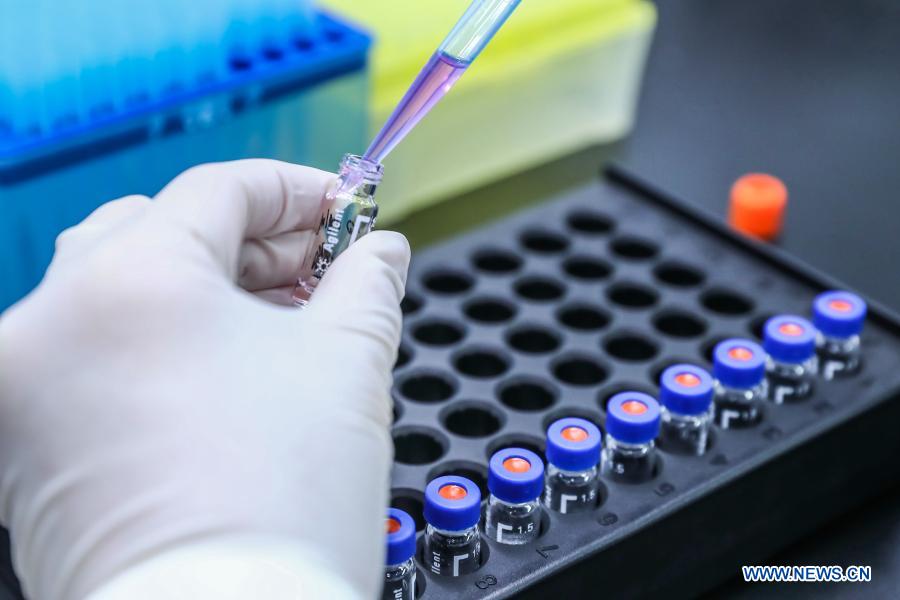
A staff member tests samples of the COVID-19 inactivated vaccine at a vaccine production base of the Beijing Biological Products Institute Co., Ltd. in Beijing, capital of China, April 11, 2020. China announced on Thursday that it had granted conditional marketing authorization for its first self-developed COVID-19 vaccine. The inactivated vaccine, which got the approval from the National Medical Products Administration (NMPA), is developed by the Beijing Biological Products Institute Co., Ltd. under the China National Biotec Group (CNBG), which is affiliated with Sinopharm. "The COVID-19 vaccines will be provided free of charge to all Chinese people," said Zeng Yixin, deputy head of the National Health Commission. (Xinhua/Zhang Yuwei)

A man receives an injection of the COVID-19 vaccine at a healthcare center in Shenzhen, south China's Guangdong Province, Dec. 30, 2020. China announced on Thursday it had granted conditional marketing authorization for the first COVID-19 vaccine. China approved the emergency use of COVID-19 vaccines in June, targeting groups with high risks of infection. By the end of November, more than 1.5 million doses of Chinese COVID-19 vaccines had been distributed for emergency use. On Dec. 15, China officially launched a vaccination program for this winter-spring period targeting several key groups. These groups include those engaged in handling imported cold-chain products, customs officers, medical workers, and people working in public transport and fresh markets. (Xinhua/Liang Xu)

A woman receives an injection of the COVID-19 vaccine at a healthcare center in Shenzhen, south China's Guangdong Province, Dec. 30, 2020. China announced on Thursday it had granted conditional marketing authorization for the first COVID-19 vaccine. China approved the emergency use of COVID-19 vaccines in June, targeting groups with high risks of infection. By the end of November, more than 1.5 million doses of Chinese COVID-19 vaccines had been distributed for emergency use. On Dec. 15, China officially launched a vaccination program for this winter-spring period targeting several key groups. These groups include those engaged in handling imported cold-chain products, customs officers, medical workers, and people working in public transport and fresh markets. (Xinhua/Liang Xu)


A man receives an injection of the COVID-19 vaccine at a clinic in Feixi County of east China's Anhui Province, Dec. 29, 2020. China announced on Thursday it had granted conditional marketing authorization for the first COVID-19 vaccine. China approved the emergency use of COVID-19 vaccines in June, targeting groups with high risks of infection. By the end of November, more than 1.5 million doses of Chinese COVID-19 vaccines had been distributed for emergency use. On Dec. 15, China officially launched a vaccination program for this winter-spring period targeting several key groups. These groups include those engaged in handling imported cold-chain products, customs officers, medical workers, and people working in public transport and fresh markets. (Xinhua/Liu Junxi)

A man receives an injection of the COVID-19 vaccine at a clinic in Feixi County of east China's Anhui Province, Dec. 29, 2020. China announced on Thursday it had granted conditional marketing authorization for the first COVID-19 vaccine. China approved the emergency use of COVID-19 vaccines in June, targeting groups with high risks of infection. By the end of November, more than 1.5 million doses of Chinese COVID-19 vaccines had been distributed for emergency use. On Dec. 15, China officially launched a vaccination program for this winter-spring period targeting several key groups. These groups include those engaged in handling imported cold-chain products, customs officers, medical workers, and people working in public transport and fresh markets. (Xinhua/Liu Junxi)

A woman receives an injection of the COVID-19 vaccine at a clinic in Feixi County of east China's Anhui Province, Dec. 29, 2020. China announced on Thursday it had granted conditional marketing authorization for the first COVID-19 vaccine. China approved the emergency use of COVID-19 vaccines in June, targeting groups with high risks of infection. By the end of November, more than 1.5 million doses of Chinese COVID-19 vaccines had been distributed for emergency use. On Dec. 15, China officially launched a vaccination program for this winter-spring period targeting several key groups. These groups include those engaged in handling imported cold-chain products, customs officers, medical workers, and people working in public transport and fresh markets. (Xinhua/Liu Junxi)


People wait for COVID-19 vaccination at a healthcare center in Shenzhen, south China's Guangdong Province, Dec. 30, 2020. China announced on Thursday it had granted conditional marketing authorization for the first COVID-19 vaccine. China approved the emergency use of COVID-19 vaccines in June, targeting groups with high risks of infection. By the end of November, more than 1.5 million doses of Chinese COVID-19 vaccines had been distributed for emergency use. On Dec. 15, China officially launched a vaccination program for this winter-spring period targeting several key groups. These groups include those engaged in handling imported cold-chain products, customs officers, medical workers, and people working in public transport and fresh markets. (Xinhua/Liang Xu)


A medical worker prepares COVID-19 vaccine injection at a clinic in Feixi County of east China's Anhui Province, Dec. 29, 2020. China announced on Thursday it had granted conditional marketing authorization for the first COVID-19 vaccine. China approved the emergency use of COVID-19 vaccines in June, targeting groups with high risks of infection. By the end of November, more than 1.5 million doses of Chinese COVID-19 vaccines had been distributed for emergency use. On Dec. 15, China officially launched a vaccination program for this winter-spring period targeting several key groups. These groups include those engaged in handling imported cold-chain products, customs officers, medical workers, and people working in public transport and fresh markets. (Xinhua/Liu Junxi)